Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study
Propolis is a bee product that has been extensively used in alternative medicine and recently has gained interest on a global scale as an essential ingredient of healthy foods and cosmetics. Propolis is also considered to improve human health and to prevent diseases such as inflammation, heart disease, diabetes and even cancer. However, the claimed effects are anticipated to be correlated to its chemical composition. Since propolis is a natural product, its composition is consequently expected to be variable depending on the local flora alignment. In this work, we present the development of a novel HPLC-PDA-ESI/MS targeted method, used to identify and quantify 59 phenolic compounds in Greek propolis hydroalcoholic extracts. Amongst them, nine phenolic compounds are herein reported for the first time in Greek propolis. Alongside GC-MS complementary analysis was employed, unveiling eight additional newly reported compounds. The antioxidant activity study of the propolis samples verified the potential of these extracts to effectively scavenge radicals, with the extract of Imathia region exhibiting comparable antioxidant activity to that of quercetin.
Introduction
Propolis is a natural product that belongs to the great family of bee products. The word propolis is a complex term originating from two ancient Greek words: pro- standing for “before or in defense” and polis meaning city. Thus, in apiculture, its meaning refers to the harboring of the hive. Propolis is a sticky, resinous substance, collected from various floral sources that are transformed and used by honeybees to construct and maintain their hives by sealing holes in their honeycombs. It is also used for smoothing out the internal walls and shelter the entrance of the hive from intruders. Trends and development in propolis research have been reviewed by Bankova [1]. In this view, the essential point in any research conducted is the chemical variability of propolis attributed to the diversity of its plant origin [2]. Propolis is a traditional remedy in alternative medicine that has been used for centuries in Egypt, Greece, and other countries as well. Propolis possesses antimicrobial [3], anti-oxidative, anti-ulcer, immunomodulatory [4] and anti-tumor activities, and the latter is proved by a plethora of reports. An informative review article on the biological activity of bee propolis in health and disease was published by Lofty in 2006 [5], collecting a significant number of research results on various medicinal aspects.
Within this context, its biological activity is attributed to its chemical composition that encompasses mainly, phenolic compounds [6]. Plant polyphenols are known for their beneficial effect on health that is vastly described for oral health (indicatively see [7]). In this regard, many research groups have presented reviews and original reports on the beneficial effect on human health of many of its constituents [8–14]. In this context, some of its components have been shown to attenuate apoptosis in rat models (for pinocembrin see [15]). Caffeic acid phenethyl ester (CAPE) is an exemplary bioactive component of propolis, exhibiting a diversity of bioactivities, such as anti-tumor effects in pre-clinical models of human breast cancer or inhibition of growth of breast cancer stem cells [16,17]. In addition, many propolis components exist in some specific plant extracts as well and have been studied for their anti-cancer activity (see indicatively [18]). The chemical base of the biological activity of flavonoids, exemplified by their antioxidant properties, are collectively presented by Kancheva and Kasaikina [19].
Until recently, only three works have dealt exclusively with the phenolic composition of propolis extracts from Greece and Cyprus. Kalogeropoulos et al. used GC-MS analysis after derivatization reaction [3] to explore its chemical profile. An additional work was published by Graikou et al., using GC-MS again, to highlight the features of Meditteranean propolis, including samples from Greece, Cyprus, Croatia and Algeria [20]. The other was reported by Lagouri et al. in 2014, including a limited amount of compounds [21]. On international scale an indicative landmark targeted study was reported by Falcao et al., incorporating almost 40 analytes, that managed to efficiently display the chemical profile of Portuguese propolis [22].
Considering the importance of propolis due to its pharmacological properties, and the limited number of works on Greek propolis, we decided to revisit its chemical composition and assess the antioxidant activity of the studied extracts. The latter was reinforced by the demographics of the European agricultural industry that render Greece second regarding bee colonies number [23], designating a substantial potential for the exploitation of this matrix.
Therefore and considering that, to our knowledge, none targeted high-performance liquid chromatographic mass spectrometric (HPLC-MS) method analysis work on phenolic compounds of Greek propolis is reported, a multi-analyte HPLC-MS (using electronspray interface and diode array, HPLC-ESI-PDA/MS) method to monitor and quantify 59 compounds belonging to relevant bioactive chemical categories, such as aromatic acids and flavonoids, was developed. The selection of compounds included in the HPLC-PDA-ESI/MS method was based on previously published works on propolis, aiming to incorporate as many as possible compounds some of which were previously not described in Greek propolis. Furthermore, Artepillin C that is a unique constituent of Brazilian propolis was incorporated, although not expected to be detected. The method was applied to the analysis of eight propolis samples from Greece and to a Brazilian tincture propolis. Tentative characterization of new compounds via HPLC-PDA-ESI/MS under full scan mode was also pursued and reported. In addition, the use of GC-MS was implemented on a complementary basis, despite its extensive use in previous works. Last but not least, the propolis extracts were assessed for their antioxidant activity using a standard protocol. Such extracts, in previous works, have been evaluated extensively for their antioxidant activity exhibiting high radical scavenging activity (indicatively see [24]). Overall, both chromatographic methods revealed several new constituents, while one of the propolis extracts displayed comparative antioxidant activity to that of the bioactive molecule of quercetin. Finally, statistical analysis demonstrated certain correlations among the variables selected.
Propolis may help treat ocular disease
The benefits of bee pollen on health have been investigated for years. Dr. Michael Cooper examines how propolis, a natural wax-like resinous substance found in beehives, can help treat ocular disease.
With a delayed spring and allergy season this year, I began to think about how to shape the global warming discussion from an objective standpoint. It is certainly a hot topic in both media and scientific circles. Essentially, our world is changing whether we like it or not.
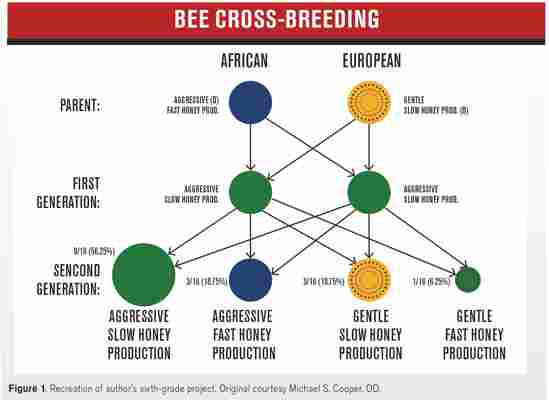
My realization of global warming started when I was in the sixth grade. My teacher Mr. Finnerty tasked the entire class with projects on how humans impact ecological change. As fortuitous as this might sound, the subject matter I tripped upon was killer bees (Figure 1).
Related: Surviving allergy season as a contact lens wearer
Bees together
In the mid- to late 1950s, the Africanized Bees program in Brazil was touted to be the perfect marriage of the gentle Western European bee (Apis mellifera-specifically Italian and Iberian) and African bee (Apis m. scutellata) to increase honey production.1,2
Although these organisms blended the organization, consistency of the former with the rapid, frenetic nature of the latter for honey production, there were two important drawbacks.
The African bee was exceedingly territorially defensive, which dominated the genetic pairing with the European bee. This trait was further enhanced with a significant lack of interest in hive mentality and the penchant to chase a person to up to 400 meters or a quarter mile away, stinging 10 times more.3,4
Parallel propolis
The parallels might not seem apparent, but the dichotomy described can be brought back to inflammation and homeostatic balance. Inflammation plays a key role in maintaining normal systemic function, but if left unchecked the process breaks down.
Bees have a known hierarchy of certain jobs, including the construction of the hive. Propolis is a natural wax-like resinous substance found in beehives used by honeybees as cement and to seal cracks or open spaces in the hive. 5
How diverse is the chemistry and plant origin of Brazilian propolis?
Honey bees are pollinators of a wide diversity of angiosperms. They seek pollen and nectar from congested inflorescences or flowers with short corolla tube, containing abundant pollen and nectar. Flowers or inflorescences of honey bee pollinated plants attract bees by their odor and bright yellow or blue coloration, often with ultraviolet absorbing nectar guides (Fenster et al. 2004). Most relationships between plants and bee pollinators are a coevolutionary and bilateral gaining process. Thus, it is a mutualistic interaction that has been fostered over the evolution of plants and insects. The characteristics associated with pollination of flowers and bees are the result of a process of coevolution that started early in the diversification of the eudicotyledons, on one side, and the Apidae, on the other (Cappellari et al. 2013). Over time, the number of bee pollinated plants increased, as well as the number of pollinator bee species. The web site Worldatlas (2018) lists 95 crop species dependent on honey bees for pollination. The total number of honey bee-pollinated wild species is unknown, but certainly it is astounding.

On the other hand, collection of resins by bee foragers is a unilateral gaining process of commensalism or parasitism, from which only the insects get advantage. There have been no attempts to determine when bees started collecting plant resins and producing propolis. Probably, this is a recent evolutionary (but not coevolutionary) process, which has evolved not in all honey bee species (Simone-Finstrom and Spivak 2010). Most likely, the number of angiosperm species foraged by honey bees for collection of propolis resin is much lower than the number of species they visit for pollen and nectar collection. Contrary to pollination, mechanisms to attract bee resin foragers have never been selected over the evolution of plant species. From the plant side, no advantage is apparent from production and provision of resin to propolis producers. On the contrary, resin collection from young vegetative tissues is somewhat similar with defoliations caused by leaf-cutter ants, although the loss of green tissues due to honey bee foragers is much lower. However, an exceptional case of mutualism between plant resin sources and honey bees has been pointed out, involving a co-evolution between stingless bees and Clusia grandiflora in South America: the floral resins play the role of nonnutritive pollinator reward for the native bees, which use them as nest construction material with effective antibacterial properties (Lokvam and Braddock 1999).
On the honey bees turn, resin collection is a difficult and time-consuming process. The proportion of total honey bee laborers collecting resins is less than 1% of the total forager work force (Borba et al. 2017). Several kinds of constraints may be pointed out as factors that magnify the burden represented by resin collection by bee laborers and narrow the diversity of plant species candidate as sources of resin for honey bee propolis.
Functional constraints
Paraphrasing Fraenkel (1959), the “raison d’être” for the investment of labor and energy toward the collection of plant resins is the benefits brought about by propolis as a barrier against enemies of the hive. The resin to be collected must be loaded with substances possessing antimicrobial activity (Simone-Finstrom and Spivak 2010; Wilson et al. 2013). It is no surprise that, despite differences in geographic locality and chemical composition, most propolis types investigated so far have been shown to exert activity against a wide diversity of microorganisms (de Groot 2013), including bacteria (Przybylek and Karpinski 2019), viruses (Fischer et al. 2007), and fungi (Oliveira et al. 2006). Also, propolis has revealed activity against honey bee parasites, such as the gut infecting microsporidium Nosema ceranae (Mura et al. 2020) and the mite Varroa infestans (Pusceddu et al. 2018). The use of plant resins by honey bees has been characterized as a self-medication process, by which propolis substances, on one side, exert detrimental effects on parasites and, on the other side, increase bee fitness and the social immunity of the hive (Simone-Finstrom et al. 2012; Borba et al. 2017; Pusceddu et al. 2018).
Factors guiding honey bees to plant sources containing biologically active resins are still largely speculative. Tropical stingless bees locate tree sources of resin using terpenes as olfactory cues (Leonhardt et al. 2010). Several types of propolis (e.g., B. dracunculifolia and poplar propolis) have mono- and sesquiterpenes and other volatile compounds in their composition (Bankova et al. 2014). Tactile stimuli have been suggested as a possible mechanism in the behavior of honey bee resin foragers (Simmone-Finstrom et al. 2010).
Most plant species are loaded with biologically active secondary metabolites. These substances have been selected over time for providing chemical defense against pathogens and herbivores (Neilson et al. 2013). Various plant species secrete highly antimicrobial resins that protect vegetative apices and young leaves, wounded tissues, etc. (Shuaib et al. 2013). Substances acting as barriers against the attack of plant enemies may be useful also as chemical defense of bees against their enemies. However, only a minority among plant species possessing these substances may be suitable sources of propolis resins to be foraged by honey bees. Next sections discuss other constraints limiting the number of plant sources of propolis resin.
Physical–mechanical constraints
Exudates on plant surfaces may turn out propolis resins that are accessed by honey bee foragers. Examples are poplar propolis, Brazilian red propolis, and Okinawan propolis (Kumazawa et al. 2008). But not all surface exudates are amenable to be collected by honey bees. Many plants have glandular hairs that release viscous-adhesive fluids, capable of entrapping and intoxicating insects and other arthropods (Jiménez-Pomárico et al. 2019). Other plants release exudates, such as gums, that are too sticky to be handled by the mouthparts of honey bee foragers. Other plant secretions have liquid or viscous texture when released but harden over time, as is the case of frankincense. So far, no propolis with resin derived from latices has been reported. Thus, a limited range of plant exudates fit conditions of being manipulated by bee foragers and then used as propolis resin.
Several secretions become available on the plant surface only occasionally. Examples are exudates that are released as a reaction to injuries caused by insect borers. In other cases, plant secretions become available during logging. The latter seems to be the case of resins of Araucaria angustifolia in south Brazil. Although diterpenes of this species have been detected in brown propolis (Bankova et al. 1996), several other papers did not detect Araucaria constituents in propolis from south Brazil. Thus, it seems that, unlike B. dracunculifolia, Araucaria is not a regular provider of resins of Brazilian propolis. A similar situation holds for Pinus trees. Logging of several cultivate north American species of Pinus is frequent in Brazil. A brown propolis from Paraná state yielded 13% of volatile oils containing mostly α- and β-pinenes, main components of pine turpentine oil (Mayworm et al. 2017). The resin of D. ecastaphyllum is a pathological secretion, being released upon injuries inflicted on the stems by borer insects. To stimulate resin release, apiculturists in northeast Brazil promote mechanical injuries to the stems, with results similar with natural injuries caused by borer insects. Only in this way D. ecastaphyllum turns out a regular provider of red propolis resin.
Alternatives to surface exudates for honey bee foragers are young tissues of plant apices. In Brazil, honey bees cut and chew fragments of young vegetative tissues of few plant species, such as B. dracunculifolia from the Cerrado and Mimosa tenuiflora from the Caatinga. Young vegetative tissues of both species are thin, almost devoid of fibers and other mechanical elements. They are also free of silica bodies on their surfaces.
Probably, many species from Cerrado and other Brazilian biomes could be sources of propolis resin, were it not for the thickness and/or hard texture of their tissues. Likely physical factors constraining bees to cut and chew plant tissues are histological components of plant tissues, such as thick cell walls and mechanical elements (fibers and sclereids). Mechanical anti-herbivore defenses play an important role in Cerrado species, as part of the syndrome of oligotrophic scleromorphism (Salatino 1993; Ribeiro et al. 1999). Often, leaves of Cerrado plants are thick, leathery, and tough, hence the designation “schlerophyllous” to them. Sclerophyllous leaves often have rigid trichomes and mechanical elements, in addition to oxalate and silicate crystals in the mesophyll. In leaves of several Cerrado species, such as Curatella americana and Davilla elliptica, siliceous phytoliths are abundant, represented by crystalline deposits within the epidermal cells and on their rigid trichomes (Lepsch et al. 2014). Phytoliths on the leaf surfaces account for the rough sandpapery feeling at the touch. Probably, the sclerophylly of many Cerrado plant species represents an unbreakable barrier to the cutting possibilities of the mandibles of forager honey bees. Actually, honey bees seem to be unable to cut even adult leaves of B. dracunculifolia, which are far from being sclerophyllous. Comparing their leaf-cutting capacity, the mandibles of the lapping-chewing mouthparts type of honey bees with the mandibles of the chewing type mouthparts of leaf-cutter ants, the former is at a far distant disadvantage relative the latter. Leaf-cutter ants are generalists, cutting fresh leaves of a wide diversity of monocotyledons and eudicotyledons. Honey bee mandibles look small and fragile in comparison with the comparatively massive mandibles of leaf-cutter ants (Fig. 14). This means that the capacity of both insects to cut and chew plant tissues is highly distinct. If honey bee mandibles were larger and their associated muscles stronger, the number of plant species exploited as sources of plant resin could be considerably higher (Salatino and Salatino 2017). Leaf sclerophylly helps understand why, among Cerrado plant species, so far only young tissues of B. dracunculifolia has been reported to be a regular botanical source of Brazilian propolis resin. The Caatinga legume species M. tenuiflora is another species with tender young vegetative tissues, which thus are amenable to the cut and chewing actions of the honey bees mouthparts (Ferreira et al. 2017). Coincidentally, B. dracunculifolia and M. tenuiflora propolis are so far the only known Brazilian propolis types with green color, which is accounted for their contents of chlorophylls derived from the aerial apical tissues of the plants.
Figure 14. Simplified representation of mouth parts of insects. (A) Chewing-lapping type (Apis mellifera); based on (accessed August 20 2020). (B) Chewing type (leaf-cutter ant; Atta sp.); based on (accessed August 20, 2020). Full size image
In this regard, the finding by Duke et al. (2017) about a brown propolis from Kangaroo Island (Australia) is enlightening. The resin is derived from a sedge (species of the Cyperaceae family) belonging to the genus Lepidosperma. Sedges are similar with grasses, in the sense that they possess leaves physically protected by sheaths of schlerenchymatic fibers. Duke et al. (2017) observed that honey bees collect resin droplets that are exuded to the leaf margins. This is the first report of propolis derived from Cyperaceae and, to our knowledge, the first report of propolis resin provided by a monocotyledon species. Monocotyledon large families, such as Poaceae (grasses), Cyperaceae (sedges), and Arecaceae (palm trees), have leaves with bundle sheaths comprising layers of schlerenchimatic fibers and silica bodies (Prychid et al. 2003). In the same Australian island is produced a different propolis, with high contents of methoxylated flavonoids (chalcones, flavonols, and dihydroflavonols), derived from exudates of pods of Acacia paradoxa (Tran et al. 2012).
Chemical-toxicological constraints
Detrimental effects on insects by plant secondary metabolites (Kortbeek et al. 2018; War et al. 2018) are likely another major constraint, narrowing the spectrum of potential sources of antimicrobial propolis resin. Secondary metabolites (also designated as specialized metabolites) have long been known to be insecticides or harmful to insects. Secondary metabolites containing nitrogen stand out as plant anti-herbivore defenses. Nicotine, an alkaloid, has been used as insecticide since 1690 (Tomizawa and Casida 2005). Glucosinolates are common and effective anti-herbivore defenses of Brassicaceae, such as cabbage and mustards (Tsao et al. 2002). Cyanogenic glycosides, detected so far in more than 2600 plant species, are toxic to arthropods (Zagrobelny et al. 2018). Hydrocyanide (HCN), a volatile compound released upon hydrolysis of cyanogenic glycosides, is toxic to aerobic organisms (van Ohlen et al. 2016). To the best of our knowledge, so far nitrogenous secondary metabolites have not been reported as constituents of honey bee propolis, although pyrrolizidine alkaloids have been detected as constituents of propolis from a stingless bee species (Coelho et al. 2015). Monocrotaline, a Crotalaria widespread pyrrolizidine alkaloid, is deterrent and toxic to honey bees (Reinhard et al. 2009). The isothiocyanates derived from hydrolysis of glucosinolates are deterrent and toxic to insects (Hopkins 2009).
Studying effects on honey bees, Detzel and Wink (1993) evaluated the attractive, deterrent, and toxic activities of substances belonging to distinct classes of secondary metabolites. Regarding nitrogenous substances, out of 22 alkaloids, 21 were deterrent, and among them, 15 were toxic; allyl isothiocyanate, a product of hydrolysis of the glucosinolate sinigrin, and amygdalin, a cyanogenic glycoside from Prunus species, were deterrent and toxic. Not only nitrogenous, but other classes of secondary metabolites were deterrent and toxic to honey bees in the mentioned experiment. The volatile oils of Lavandula, Melissa, Rosmarinus, Syzygium, and Thymus were deterrent and toxic; the volatile oil of Citrus was attractive, but nonetheless toxic. Coumarins are phenolic compounds, several of them toxic to vertebrates and insects (Sarker and Nahar 2017). Three coumarins—coumarin itself, aesculin, and umbelliferone—were shown to be deterrent and toxic to honey bees; in addition, a saponin and a sample of tannin were also deterrent and toxic (Detzel and Wink 1993).
Saponins are glycosides with a triterpenoid or steroidal nucleus, having deterrent and toxic effects on insects (De Geyter et al. 2007; Chaieb 2010). Tannins are phenolic polymers that bind strongly with proteins and may be toxic due to oxidative stress they cause on herbivores (Salminen and Karonen 2011; Barbehenn and Constabel 2011). Saponins and tannins are widespread in vascular plants, and thus several propolis types probably contain at least detectable amounts of these secondary metabolites (Mayworm et al. 2014). High tannin content is common in plant species of Cerrado and is associated with sclerophylly (Madeira et al. 1998), while saponins are abundant in many species of Cerrado and other biomes.
The results of Detzel and Wink (1993) are relevant to reveal the amplitude of classes of secondary metabolites that may be detrimental to honey bees. Out of 39 allelochemicals tested, most exhibited deterrent effect, among which 17 exerted some degree of toxicity. It is interesting to notice that, among the classes of metabolites tested, flavonoids were almost devoid of negative effects. Among six flavonoids tested, only catechin (a monomer constituent of condensed tannins) had deterrent and toxic effect. All other flavonoids showed no effect in the feeding tests. This observation is coherent with their utility by honey bees, derived from their known antimicrobial and immune stimulating effect, with no collateral toxic effects. Detzel and Wink (1993) tested the effects of substances dissolved in nectar, a substance that is engorged by worker bees and treated by enzymes in the crop or honey stomach (Sammataro and Cicero 2010). The resin is not ingested by foraging bees, so that the two foraging processes (nectar and resin collection) cannot be placed side by side regarding toxicity. On the other hand, they are comparable regarding deterrence, so that resins containing unpalatable and potentially toxic substances, such as bitter alkaloids and saponins, as well as astringent tannins, are likely avoided by foraging workers.
The above comments about the non-toxicity of resins collected and manipulated by honey bees is an argument in line with the common claim that propolis is safe for human consumption. Propolis is assumed as a non-toxic product, in part due to experiments of toxicological effects of flavonoids, carried out with mice. It was noted that the toxicity of flavonoids to mice is extremely low, values of LD 50 ranging from 8000 to 40,000 mg/kg (Havsteen 1983).
Brazilian biomes: plant megadiversity vs. narrow diversity of propolis plant sources
Brazil is a country with continental expanse, biome diversity (Fig. 2), and a recognized plant megadiversity (Canhos et al. 2015). One of the first themes we learn at school about plant-animal relationships is pollination and the wide diversity of bee-visited flowers. At a first evaluation, one is led to imagine that a similar picture should hold regarding the interaction between bees and plant sources of resins. Therefore, it is normally expected to exist a wide diversity of plant sources of Brazilian propolis. In fact, this assumption is implicit on comments of authors in many papers of propolis literature. The opposite picture, the idea that only a narrow diversity of Brazilian plant species is suitable as resin sources for propolis production, is counterintuitive. Contrary to the logical reasoning based on taxonomic megadiversity and plant chemical plurality (Sen and Samanta 2015), results of the first estimation of the number of propolis types in Brazil came out with only 12 types, upon examination of nearly 500 samples of propolis, covering a wide expanse of the Brazilian territory (Park et al. 2000). Not many types of Brazilian propolis and corresponding plant sources have been later characterized, such as the mangrove red, the Amazonian black, and the Caatinga green types (Table 1, Fig. 2). Given all the requisites that a plant must fulfil to be become resin providers of propolis, it comes not as a surprise the low number of species that are effective plant origins of Brazilian propolis.
Much is still necessary to get a wider and more realistic magnitude of the diversity of Brazilian propolis types. New chemically distinct types are expected to be characterized, along with corresponding plant origins. Honey bees have high adaptive capacity and are efficient explorers of environmental resources. For example, if challenged to grow in the absence of their preferred plant source of propolis resin (B. dracunculifolia) resin, honey bee hives managed to find new sources (Piperaceae plants; Fernandes-Silva et al. 2020). With time, undoubtedly the list of plant sources on Table 1 will be considerably expanded. The prospect, however, is that the list will stand within a limited range, far below the diversity of honey bee pollinated plant species.