Identification of Catechol as a New Marker for Detecting Propolis Adulteration
Adulteration of propolis with poplar extract is a serious issue in the bee products market. The aim of this study was to identify marker compounds in adulterated propolis, and examine the transformation of chemical components from poplar buds to propolis. The chemical profiles of poplar extracts and propolis were compared, and a new marker compound, catechol, was isolated and identified from the extracts of poplar buds. The polyphenol oxidase, catechol oxidase, responsible for catalyzing oxidation of catechol was detected in poplar buds and propolis. The results indicate catechol can be used as a marker to detect propolis adulterated with poplar extract.

1. Introduction
The honeybee is a perennial species that exploits virtually all habitats on Earth, and their evolutionary and existent success are not only because of collecting nectar and pollen for fulfilling their nutritional needs, but also the ability to produce bee products: beeswax, venom, propolis and royal jelly [1,2]. As a case of ‘self-medication’ by the bee colonies, propolis has a role in the social immunity of honeybee, reducing the risk of disease and parasite transmission through the colony [3]. Since the ancient time, propolis has been used as a folk medicine for human health and preventing diseases, and it is gaining wider acceptance in popular medicine all over the world. A great number of studies have focused on the pharmacological and biological properties of propolis, including anti-inflammation [4], anticancer [5], antioxidative [6], immunomodulatory [7], antimicrobial, antibacterial, antiviral, and antifungal effects [7,8].
The materials available to bees for manufacturing propolis are plant buds, and substances actively secreted by plants as well as substances exuded from wounds in plants, but bees have a marked preference for one or a few sticky sources [3,9]. According to different geographical locations, poplar, conifer, birch, pine, alder, willow, palm, Baccharis dracunculifolia and Dalbergia ecastaphyllum are identified as the plant sources of propolis [10,11]. Among a multitude of botanical sources, Populus species are considered as the main plant origin of propolis all over the world, especially in the temperate zone [3]. Most propolis collected from Europe, North America, template Asia, has a similar chemical composition, color and smell as the extract of Populus bud. The yield of propolis is relatively low, and does not meet the demand of the growing market, leading to the adulteration of propolis with the extract of the poplar buds. This counterfeiting behavior has seriously disrupted the propolis industry, raising concerns on the quality, efficacy and safety of fake propolis.
A number of studies have been carried out to compare the chemical compositions of propolis and poplar extract. It has been shown that the flavonoids in these two natural products are very similar. Wu et al. found the differences between propolis and poplar extract are caused by the amounts of long-chain alkyl compounds [12]. Zhang et al. developed a HPLC method to use salicin to screen counterfeit propolis [13]. However, salicin is susceptible to acid and can be hydrolyzed to glucose and saligenin which are not detectable by the method. Therefore a new marker compound for distinguishing propolis from poplar extract is urgently needed.
The original plant resin can be modified by enzymes from honeybee [14], which leads to the differences of the chemical constitutes between the propolis and its botanical sources. For example, β-glucosidase has been purified from ventriculus, honey sac, and hypopharyngeal glands of Apis mellifera [15], and propolis. It hydrolyzes flavonoid mono-glucosides in plant resins during propolis collection and processing [16,17].
Catechol (o-diphenol, ) occurs naturally in fruits, vegetables and plants, along with polyphenol oxidase (PPO), an enzyme localized on the thylakoids of chloroplasts, in vesicles or other bodies of non-green plastid types [18]. PPO catalyzes two different reactions: hydroxylation of monophenols to o-diphenols and oxidation of p- and o-diphenols to p- and o-quinones [19]. The specific isozyme which works on o-diphenol substrates such as catechol is catechol oxidase (EC 1.10.3.1). Upon mixing polyphenol oxidase with the substrate in exposure to oxygen, the colorless catechol is oxidized to reddish-brown melanoid pigments o-diquinones, derivatives of benzoquinone [20].
In our previous studies, we reported that in addition to salicin, there was an unknown chemical component (shown as peak A in ) in poplar exact (gum) but not in propolis samples [13]. The aim of this study was to identify this marker compound to detect the adulteration of propolis. In addition, we also studied the presence of polyphenol oxidase, which may be a possible reason catechol is not present in propolis.
Processing Propolis: Part 1
by: Ross Conrad
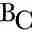
The crisp cool Autumn weather arrives at a time when the color of the leaves change, mice build nests in well-protected warm places, and bees finish plugging up the cracks in their hives with propolis in anticipation of Winter.
The term propolis, (aka bee glue) originated with the Greeks who often observed a sticky resinous substance around the entrance to their hives. In Greek, “Pro” means coming before or in front of, and “Polis” is the Greek word for city or a body of citizens. Thus, propolis is what one could expect to find at the entrance to the city of the bees. Today beekeepers will often observe that the bees will use propolis to restrict or narrow the entrance to the hive to make it easier to defend. Honey bees use propolis as both a building material and as a way to sterilize and disinfect the cavity that contains the colony. This is because, as we will explore in this two part series, propolis is among the most powerful antimicrobial substances found in nature.
Honey bees make propolis out of the resins they collect from deciduous trees such as cottonwood, birch, alder and poplar (aspen). As these trees bud, they exude these resins around the bud in order to protect is from fungi and other diseses. Foraging bees utilize their pollen baskets (corbicula) to carry globs of propolis resins back to the hive. Unlike with pollen however, foragers require the help of other bees within the colony to help them remove the sticky resins from their hind legs so it may be used by the colony.
Composition
Over 240 compounds have been reported to have been extracted from honey bee propolis. While the composition of propolis will differ somewhat depending on which trees the bees gather the resins from, the typical composition tends to be approximately 45-55% resins, 25-35% waxes and fatty acids, 10% essential oils and aromatic compounds (phenolics), which includes vanillin and gives propolis the wonderful vanilla-like smell, and 5% pollen. An additional 5% or so of the constituents of propolis are other organic compounds such as flavonoids (or bioflavonoids collectively known as Vitamin P and citrin). There are even minor components of propolis that researchers have, to date, been unable to identify at all. When warm, propolis is as sticky as chewing gum, but it becomes hard and brittle when cold.
Production
Research indicates that conditions which stimulate the collection of propolis by a colony of bees include: rough surfaces within the hive, cracks and crevices within the hive that are smaller than the 5/16th of an inch bee space and not suitable for building comb, drafts and light coming into the hive in unwanted places, and disease infections.
Beekeeping supply companies often sell traps that can be used to collect propolis. The trap consists of a thin plastic sheet that has narrow slits cut into it and replaces the inner cover on the hive. Over time bees will fill the narrow slits in the plastic when the outer cover is flat on the hive, however, leaving the outer cover propped up to allow light and air in through the top of the hive will encourage the bees to plug up the holes in the propolis trap faster.
Once the trap is plugged up with propolis it is put in a bag and placed in a freezer for at least a few hours. Immediately upon removal from the freezer the trap (still inside the bag) is banged against a hard surface such as a table top, or simply contorted and gently bent back and forth in order to cause the brittle propolis to crack, break and fall from the trap.
Propolis can be collected by catching the hive scrapings when cleaning out the honey supers during the honey harvest as well. Unlike propolis collected from a trap, hive scrapings will tend to contain contaminants such as bits of wax, wood, dead bees, etc. One way these contaminants can be removed from the propolis by soaking the scrapings in a pail of water. Dead bees, pieces of wood and bees wax will tend to float while the propolis will tend to sink, allowing the beekeeper to separate out the majority of contaminants.
Another way to clean propolis hive scrapings is to place the propolis scrapings into an oven-proof container. The scrapings are covered with two to three inches of water and placed in an oven at 200°F. The contents of the container should be baked for at least two hours and stirred often in order to release any wax that may be trapped within the mass of propolis. The melted wax, pieces of wood, etc., will float to the surface of the water while the propolis will stick to the bottom of the container. After the container is removed from the oven and cooled, the waxy layer on the surface of the water can be removed and the water carefully poured off to reveal the colored propolis mass beneath it. The container of propolis can then be frozen and when the propolis is brittle, it can be chipped out of the container. The cleaned propolis pieces should be spread out on a sheet of paper or cardboard to dry before placing then into storage.
As noted, honey bees use propolis as an extension of their immune system and rely upon it to keep healthy. I believe this is one of the primary reasons that when the genome mapping of the honey bee was conducted, it was found that bees have far fewer genes dedicated to immune response than any of the other insects that had also undergone genome mapping. The utilization of propolis by the colony appears to have eliminated the need for bees to invest biological energy in the development of a more robust immune system within the body of each bee. As a result, I don’t like to take propolis from my hives since doing so may decrease the overall health and vitality of the bees. However as we shall see, the profound health benefits that propolis can provide for humans compels me to collect propolis, but typically only from the honey supers that are being harvested in the honey house. Additionally, since I don’t want to do the extra work of cleaning it, I only collect and put aside chunks of pure propolis rather than try to capture all the propolis in the scrapings, etc. As a result, the amount of propolis I produce annually is rather small and results in only two-to-three dozen bottles of propolis alcohol tincture.
Processing
While propolis can be found in many products from toothpaste and skin creams to healing salves, herbal tinctures, syrups and elixirs, propolis does not require any processing (other than cleaning) in order for it to be used. For gum, dental, or sore throat issues simply tuck a chunk of raw propolis between the gum and cheek and suck on it. This is the simplest way to use it, although its benefits may be limited and it may stick to your teeth if you are not careful. Here are some of the more usual commercially available forms that pure processed propolis can be found in.
Powdered in capsule or tablet form
Before turning the propolis into powder, it must be cleaned such as described above. One way to turn chunks of propolis into powder is to freeze it for at least a few hours, along with a hand grinder or mortar and pestle. As soon as the propolis and grinder are removed from the freezer, the propolis should be ground up. A hand-powered grinder is preferable to an electric grinder since a hand grinder will not heat up as fast when in use, reducing the speed with which the propolis will become sticky and gum up the grinder.
While large companies may make tablets out of propolis, small producers may purchase capsules that can be used to encapsulate the powdered propolis. Alternatively, powdered propolis can be mixed with raw honey to make an especially tasty medicine. Since the propolis will tend to float to the top of liquid honey, it is best to use honey that is either naturally crystalized or in the process of crystalizing in order to capture and keep the propolis suspended in and throughout the honey.
Tinctures or Extracts
It is relatively easy to make propolis extracts or tinctures and they can be prepared with minimal time investment with only two ingredients: propolis and an appropriate solvent.
Choosing the correct solvent is very important, especially if the product is to be used for human consumption. For the highest quality extract, ethanol (also called grain alcohol, pure alcohol or ethyl alcohol C 2 H 6 O) is used, however any food grade alcohol that is at least 130 proof (65% alcohol) will work well.
Ideally propolis is ground into a powder prior to making a tincture or extract from it. This is done in order to maximize the surface area of the propolis for the solvent to work on. I have found however, that large unbroken chunks of propolis will dissolved when using grain alcohol to make tinctures, eliminating the work of grinding up the propolis beforehand.
To make the tincture, place the alcohol and propolis into a water-tight container, seal the top and shake briefly. Shaking should be repeated once or twice a day, and the mixture left soaking in the alcohol for one-to-two weeks for best results.
After a couple weeks, the extract is ready and may be filtered through a clean and very fine cloth, paper filter or cotton ball. The remains of the first filtration can be soaked in alcohol again for an additional extract, however it may not be quite as potent as the first extract.
The finished tincture will be a clear liquid, free of particles and dark brown or slightly reddish in color. It is best bottled in clean, dark, airtight bottles for long-term storage. When a tincture with a high concentration of propolis is desired one can simply add more propolis and less solvent to the container and the filtering process can be eliminated. Rather than filter the resulting liquid, the extract is simply poured off allowing some of the fine particles that do not fully dissolve and collect in the bottom of the soaking container to be transferred into the final storage container. Additionally, the container of the final propolis tincture can be left out with a porous cover (such as cheese cloth) in order to allow some of the solvent to evaporate over time increasing the percentage of propolis in the final product and reducing the ethanol content.
Denatured, rubbing or methyl alcohol should never be used if the final product is intended for internal human consumption since toxins are added to such alcohols in order to make them unpalatable and prevent ingestion. To be safe, the only time that rubbing alcohol may be used to produce a propolis tincture is when the resulting tincture is to be applied to beekeeping equipment in order to ‘pre-propolize’ it for honey bee use, as is done by some biodynamic beekeepers. However since there are different types of denatured alcohols intended for different purposes and the chemicals added to denature alcohol may be poisonous to bees, caution should be taken and such extracts should only be used to preserve the outside of the hive, reserving only extracts appropriate for human consumption for use on the inner surfaces of hive bodies and supers.
Aqueous (Water) or Hydrolyzed Propolis
For individuals that wish to avoid alcohol, a water extract may be desirable. Aqueous extracts can be obtained by soaking propolis in water or boiling it in water. When boiling, some of the propolis’ aromatic compounds may be lost however. Even though the yield of active and medicinal ingredients is generally lower than when making an alcohol tincture, water extracts have been shown to have powerful bactericidal and fungicidal effects. All other processing, filtering, etc. is the same as described above. Water extracts of propolis should be refrigerated in order to suppress the growth of mold.
Oil Extract
An oil extract of propolis may be obtained by filling a pot with propolis and any food grade oil (coconut, sunflower, olive, etc.) or even with butter. The contents of the pan are gently heated in a water bath and stirred constantly for about 10 minutes. The resulting extract can be filtered and stored in sealed containers in the dark.
Propolis extracts are also obtained by using other solvents such as vegetable glycerin or Propylene Glycol. Even though the alcohol process results in the most potent extract, it does not mean it will be the best for all uses and situations. For example, water extracts are preferred when treating eye infections since it is not advisable to put alcohol or oil in the eyes for obvious reasons. Oil extracts on the other hand are recommended for mouth and gum problems, and may be best for external use on people or babies with sensitive skin.
Next month we will explore the many medicinal benefits and uses of propolis for man and animals.
Ross Conrad is the author of Natural Beekeeping: Organic Approaches to Modern Apiculture, 2nd Edition.
Full article: Standard methods for Apis mellifera propolis research
Propolis consists of plant resins and beeswax and the chemical analysis of propolis is directed to the plant derived compounds as they are the components responsible for the bioactivity of propolis. The compounds also indicate the plant(s) that bees have visited for resin collection. The chemical information is important with respect to quality control and standardization purposes. Also, if the propolis type is new and unexplored, it may contain new valuable bioactive compounds.

Separate the insoluble portion by filtration; keep the ethanolic solutions in a freezer at −16 °C overnight and filter again at this temperature to reduce the wax content of the extracts.
Extract ground propolis by maceration for 7 days in an orbital shaker at a temperature of 30 °C, with 10 ml of absolute ethanol (Merck; Darmstadt, Germany) for every 3 g of crude propolis.
The obtained extract can be evaporated to dryness for further use or used as is in further experiments. Alternative extraction procedures might be applied depending on the analysis for which the propolis extract is to be used. For biological tests, a variety of solvents have been used, including methanol, different ethanol-water mixtures (80, 90, and 96%), absolute ethanol, glycerol, water (Park & Ikegaki, 1998 ; Sforcin & Bankova, 2011 ), and even DMSO (Netíková, Bogusch, & Heneberg, 2013 ). It is important to note that water dissolves less than 10% of the weight of propolis.
The concentration C of the extract the amount of propolis) is determined by evaporating 2 ml of the extract to dryness in vacuo to constant weight g and using the formula C = g /2 mg/ml (average of three replicates).
Filter the resulting suspension at room temperature using a paper filter and repeat the procedure with the part trapped in the filter, extracting the residue again under the same conditions. Experiments have shown that a third extraction under the same conditions is not necessary since the third extract yielded a negligible amount of dry propolis (Popova et al., 2004 ).
Measure a sample of the powdered propolis, add 70% ethanol (1:30 w:v) and keep it for 24 h at room temperature. Alternatively, sonicate the suspension (propolis in 70% ethanol) for 20 min in an ultrasonic bath at 20 °C.
Keep propolis overnight in a freezer (−20 °C). Powder the frozen propolis using a coffee mill or other similar grinding device to achieve a particle size of about 10–80 μm.
The aim of the extraction is to remove the major plant secondary metabolites from any impurities, such as beeswax, for further analysis or for biotests. This is achieved by extraction with 70% ethanol, as noted below.
Dry the organic layer over anhydrous Na 2 SO 4 : add 3 g of anhydrous Na 2 SO 4 , shake the flask for 5 min and filter the liquid using a filter paper. Wash the solid on the filter with 1 ml ice cold n -pentane - diethyl ether 1:1 (v/v).
After the distillation is over, remove the water layer using a separatory funnel. Keep the organic layer in refrigerator until further processing.
Propolis volatile constituents are responsible for the specific pleasant aroma of propolis and contribute to its biological activity, although their amount is seldom greater than 1% of the weight of the sample. They also may play an important role as olfactory cues during resin collection by honey bees (Leonhardt, Zeilhofer, Bluthgen, & Schmitt, 2010 ). Different methods have been used to extract propolis volatiles: steam distillation, hydrodistillation (Clevenger), distillation-extraction (Likens-Nikerson), solvent extraction (including ultrasound-assisted and microwave-assisted extraction), and static and dynamic head-space, solid-phase microextraction. The method of extraction significantly affects the chemical composition of the volatile constituents of propolis (Bankova, Popova, & Trusheva, 2014 ). Here, we describe one of the most often used approaches for propolis volatile extraction, distillation-extraction (Bankova, Boudourova-Krasteva, Popov, Sforcin, & Funari, 1998 ). A review of volatile extraction procedures for hive components in general can be found in Torto et al. ( 2013 ).
where Σ A i is the sum of all the peak areas in the chromatogram. Thus, the percentage of the individual compounds refers to percent of the Total Ion Current (TIC), and the result should not be considered as quantitative in absolute terms (IOFI Working Group on Methods of Analysis, 2011 ).
The quantification of individual constituents is based on internal normalization. This is a general approach used in cases where it is impossible to use other methods such as the internal standard method. The internal normalization method is based on the assumption that all detector response factors are unity, and the following equation should be applied: % Analyte = A a ∑ A i × 100
The identification of individual compounds (such as trimethylsilyl derivatives) can be performed using computer searches on commercial libraries (such as NIST 14, Wiley 10, etc.), comparison with spectra and retention characteristics of authentic samples, and literature data. If no reference spectra are available, identification can be performed based on the characteristic mass-spectral fragmentation, in such cases the compounds are described as “tentative structures”.
The GC conditions can vary depending on the apparatus used and with respect to optimization of chromatographic separation (Cheng, Qin, Guo, Hu, & Wu, 2013 ; Kaškonienė, Kaškonas, Maruška, & Kubilienė, 2014 ; Nunes & Guerreiro, 2012 ).
Use a 30 m long, 0.25 mm ID, and 0.25 μm film thickness SPB-1 capillary column. Other columns with similar characteristics can be also used depends on analytical needs.
The GC–MS analysis should be performed with a proper instrument such as a Hewlett–Packard gas chromatograph 5890 series II Plus linked to a Hewlett–Packard 5972 mass spectrometer system (Bankova et al., 1998 ).
The GC conditions can vary depending on the apparatus used and with respect to optimization of chromatographic separation (Isidorov, Szczepaniak, & Bakier, 2014 ).
Use a 30 m long, 0.25 mm ID, and 0.5 μm film thickness HP5-MS capillary column. Other columns with similar characteristics also can be used depending on analytical need.
The GC–MS analysis should be performed with a proper instrument such as a Hewlett–Packard gas chromatograph 5890 series II Plus linked to a Hewlett–Packard 5972 mass spectrometer system (Trusheva et al., 2011 ).
Dry propolis extracts obtained according to Section 3.1.1 are analyzed by GC-MS after derivatization. The derivatization (conversion to trimethylsilyl derivatives) is performed, as follows:
Prior to the GC-MS analysis, derivatization of the propolis extracts is required because propolis contains metabolites that are not volatile enough for gas chromatography (Greenaway, Scaysbrook, & Whatley, 1987 ). One of the most widely used derivatization reagents is N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) (Bankova, Dyulgerov, Popov, & Marekov, 1987 ; Greenaway & Whatley, 1990 ). Silyl derivatives (trimethylsilyl ethers) obtained from propolis are less polar and more volatile than their parent compounds and are suitable for analysis by GC-EIMS (gas chromatography – electron impact mass spectrometry).
Gas chromatography-mass spectrometry (GC-MS) is one of the so-called hyphenated analytical techniques extensively used for the chemical analysis of complex mixtures such as propolis. GC-MS combines the features of gas chromatography for compound separation and mass spectrometry to identify different substances. This method is used for chemical profiling of propolis for the needs of comparative analysis, quality control and standardization.
The fast technical evolution of the LC-MS systems, particularly in respect to the mass analyzers, will continue to allow new findings within the chemical composition of propolis.
Compare the UV spectra and MS information to those of reference compounds. If standards are not available, the identity of the compounds can be achieved through comparison of the product ion spectra and retention times with pure compounds isolated from propolis or combining UV data with MS fragmentation patterns previously reported in the literature (Section 3.4.3 ).
Chose the right LC parameters for the analysis such as a reversed-phase C18 HPLC column, which is the most selective in propolis analysis (Section 3.4.2.1 ).
LC-MS is a powerful tool that can be used to overcome the difficult task of propolis chemical profiling, due to the high diversity of the resin floral sources collected by honey bees. To enhance the amount of structural information given by the technique, the most important features to be considered in LC-MS propolis chemical profiling are to:
Attention has to be taken to experimental conditions used, such as the type of ion source and mass analyzer, when comparing literature data, since different fragments can be found when different experimental set-up and/or operating conditions are applied. The mass spectra of flavonoids obtained with quadrupole and ion-trap instruments typically are closely similar, even though relative abundances of fragment ions and adducts do show differences. Therefore, direct comparison of spectra obtained with these two instruments is allowed. The main advantage of an ion-trap instrument is the possibility to perform MS n experiments (Steinmann & Ganzera, 2011 ).
Fragmentation patterns are specific for a given compound or class of compounds. For example, for the negative ion mode, phenolic acids demonstrated a common fragmentation pattern, with a loss of the carboxyl group (CO 2 , −44 Da) (Falcão et al., 2010 ). In the case of flavonoids, the distinct flavonoids classes differ in their pattern of substitution, which strongly influences the fragment pathway, the interpretation of MS/MS data provides specific structural information about the type of molecules. The MS 2 spectrum of many of these flavonoids (Table 2 ) revealed the fragments at m/z 151 or at m/z 165, which are resultant from the retro Diels-Alder mechanism (Cuyckens & Claeys, 2004 ). Also, neutral losses commonly described to occur in these compounds, such as the small molecules CO (−28 Da), CO 2 (−44 Da), C 2 H 2 O (−42 Da), as well as the successive losses of these molecules, were also observed (Cuyckens & Claeys, 2004 ). In accordance with Cuyckens and Claeys ( 2004 ), methylated flavonoids presented a significant [M-H-CH 3 ] −• product ion.
The structural elucidation of different classes of propolis compounds is achieved by comparing their chromatographic behavior, UV spectra and MS information, to those of reference compounds. When standards are not available, the identity of the compounds can be achieved through comparison of the product ion spectra and retention times with pure compounds isolated from propolis or, alternatively, combining UV data with MS fragmentation patterns previously reported in the literature (Falcão et al., 2013a ). Table 2 shows the UV data and MS fragmentation of many compounds described in the literature as propolis constituents. Only compounds with all the information regarding MS fragmentation are present.
Propolis chemical composition is a rich pool of phenolic compounds. Those, often referred to as polyphenols, embody a class of widely distributed and chemically diverse secondary metabolites synthesized in plants at different developmental stages (Steinmann & Ganzera, 2011 ). Polyphenols possess at least one aromatic ring with one or more hydroxyl functional groups. Flavonoids, whose structures are based on a C6-C3-C6 skeleton, are the most abundant group of phenolic compounds, and are sub-divided into several classes differing in the oxidation state of the central heterocyclic ring (Veitch & Grayer, 2008 ). These comprise chalcones, flavones, flavonols, flavanones, isoflavonoids, anthocyanidins and flavanols (catechins and tannins). Non-flavonoids comprise simple phenols, phenolic acids, coumarins, xanthones, stilbenes, lignins and lignans. Phenolic acids are further divided into benzoic acid derivatives, based on a C6-C1 skeleton, and cinnamic acid derivatives, which are based on a C6-C3 skeleton (Veitch & Grayer, 2008 ). The variability of propolis chemical composition contains large numbers of phenolics from different classes including, unexpectedly, glycoside phenolic compounds, clearly highlighting the challenges associated with their analysis.
Concerning the mass analyzers, the ion trap is the one most recommended for the profiling of propolis composition since it is specially designed for multiple fragmentation steps (MS n ). Regarding target analysis, a tandem-MS detection over a single-stage MS operation is recommended because of the much better selectivity and the wider-ranging information that can be obtained (de Rijke et al., 2006 ). In linear ion traps, ions are isolated and accumulated due to a special arrangement of hyperbolic and ring shaped electrodes as well as oscillating electric fields. Then the ions can be fragmented by collision-induced decomposition (CID) (Ignat et al., 2011 ). The MS n data is simultaneously acquired for the selected precursor ion. The collision induced decomposition (CID)–MS–MS and MS n experiments should be performed using helium as the collision gas, with collision energy (CE) of 20–40 eV. The CE is dependent on the molecule stability under study. In the negative ion mode, collision energies of 20 eV for phenolic acids and 20–40 eV for flavonoids are suitable (Pellati et al., 2011 ).
The ion source used should be electron-spray ionization (ESI). ESI is a soft ionization technique for a wide range of compounds (slight fragmentation but adducts are often observed), where ionization is achieved by applying a high electric charge to the sample needle, with voltage between 3 and 5 kV and the capillary temperature between 300 and 350 °C. ESI can be operated in the negative or positive full scan ion mode, although, and concerning the phenolic compounds, a higher sensitivity and better fragmentations can be achieved with the negative ion, thus resulting in more structural information (Cuyckens & Claeys, 2004 ). A more recent development is atmospheric pressure photoionization (APPI). If the compounds are poorly ionized by ESI and APCI, APPI should be considered as an alternative (Ignat, Volf, & Popa, 2011 ).
Given the unique characteristics of different mass spectrometers, it is critical to choose the suitable MS parameters. Table 1 summarizes the best conditions for the MS analysis of propolis phenolic compounds.
The eluent is composed of a binary solvent system containing acidified water (solvent A) combined with a polar organic solvent (solvent B). Gradient elution has usually been mandatory in recognition of the complexity of the propolis chemical profile. 0.1% formic or acetic acid can be added to water (as solvent A) and acetonitrile or methanol (as solvent B) are commonly used in propolis analysis. 0.1% formic acid is the most suitable when using a MS detector. The flow rate is dependent on the type of column used, but for the above parameters it is recommended to be 1 ml min −1 . Temperature control of the column should also be considered to achieve a better peak separation, between 25 and 40 °C, with 30 °C being the most suitable for propolis compound separation. For a flow rate of 1 ml min −1 , a post-column split of 0.2 ml min −1 to MS should be applied (Falcão et al., 2013a ).
The chromatographic conditions of the HPLC methods include, almost exclusively, the use of UV–Vis diode array detector (DAD) with spectral data for all peaks acquired in the range of 200–600 nm, although 280 nm is the most generic wavelength for phenolic compounds due to the high molar absorptivity of the different phenolic classes at that wavelength.
A fast and ultra-fast separation can be achieved with columns packed with sub-2 μm particles operating at ultra-high pressure systems. Ultra-high-performance liquid chromatography (UHPLC) is quite versatile and can be used to increase throughput, particularly suitable for the analysis of complex samples such as plant extracts or their metabolites (Nicoli et al., 2005 ). Recent work has been performed with propolis in equivalent columns of Waters BEH C18 (50 mm × 2.1 mm ID × 1.7 μm particle size) reducing the time run to 12 min (Novak et al., 2014 ).
Reversed phase HPLC is doubtlessly the most widely used chromatographic method in propolis analysis (Falcão et al., 2010 ; Gardana, Scaglianti, Pietta, & Simonetti, 2007 ; Pellati, Orlandini, Pinetti, & Benvenuti, 2011 ; Piccinelli et al., 2011 ; Righi, Negri, & Salatino, 2013 ; Volpi & Bergonzini, 2006 ). Most appropriate are octadecylsilane columns (ODS or C18). Nucleosil C18 250 × 4 mm ID, 5μm particle diameter (Falcão et al., 2010 ); Luna C18 column 150 × 2.0 mm ID, 5 μm (Piccinelli et al., 2011 ); and CLC-ODS 150 × 6.0 mm ID (Midorikawa et al., 2001 ) can also give good results. Due to the complex nature of the matrix, a drawback for the use of these columns is the long runs needed, frequently above 50 min per run.
HPLC separation is largely dependent on the different affinities between the propolis compounds and the stationary phase. For a particular application, the chemical properties of the packing and physical properties of the column (e.g. particle size and column dimensions) need to be taken into account.
The following sub-Section describes in detail the parameters for LC and MS that could be applied for the analysis of propolis.
The use of LC–MS for the qualitative and quantitative analysis of constituents in propolis has increased steadily over the last years.
High performance liquid chromatography (HPLC) was and still is the preferred separation technique for the analysis of natural products (Steinmann & Ganzera, 2011 ). Recent developments of new stationary phases and pumping devices enabling pressures up to 1300 bar are further supporting this trend (Steinmann & Ganzera, 2011 ). Different detectors can be used, depending on the analytes investigated. The most commonly used detectors for analyzing propolis are DAD and MS detectors.
The relatively polar nature of propolis constituents (with several hydroxyl groups in their structure), combined with soft ionization techniques compatible with liquid chromatography, make HPLC-DAD and LC-MS the favorite methods for analysis of propolis balsamic content (Sforcin & Bankova, 2011 ). In the structural identification of new compounds, both mass spectrometry with electrospray ionization (ESI-MS) in the negative (Falcão et al., 2010 ) or positive ion mode (Piccinelli et al., 2011 ) studies are satisfactory.
MS fingerprinting may be applied to propolis samples to characterize their composition, identify the plant sources, and indicate their potential therapeutic application. Besides ESI, a new ionization source, named easy ambient sonic ionization (EASI), has been used for this purpose as well (Sawaya et al., 2010 ). The use of chemometric methods such as PCA to analyze the results is frequently necessary due to the large number of ions observed in each spectrum. The results of the analyses are capable of grouping similar samples, indicating their marker ions and, in some cases, correlating with the biological activity of samples.
Using the same extraction and analysis procedures, propolis samples can be compared to the plant sources of their resins. This could allow one to link the resin producing source plant to the propolis from these regions (Marcucci, Sawaya, Custodio, Paulino, & Eberlin, 2008 ).
Figure 4. Genaral process used in ESI-MS fingerprinting studies: ionization and anlaysis by ESI-MS, extraction of the m/z and intensity of selected ions, statistical analysis of the data via PCA to group samples and indicate the marker ions for each group.
Figure 4. Genaral process used in ESI-MS fingerprinting studies: ionization and anlaysis by ESI-MS, extraction of the m/z and intensity of selected ions, statistical analysis of the data via PCA to group samples and indicate the marker ions for each group.
Samples are grouped according to their geographic origin (Sawaya et al., 2004 ). Furthermore, tandem mass spectrometry with collision induced dissociation (CID) allowed on-line structural identification of certain marker ions such as dicaffeoylquinic acid, 3,5-Diprenyl-4-hydroxycinnamic acid, Pinocembrin, Chrysin, 3-Prenyl-4-hydroxycinnamic acid, 2,2-Dimethyl-6-carboxyethenyl-2H-1-benzopyran and p -Coumaric acid (Sawaya et al., 2004 ). The general flow of these ESI-MS fingerprinting studies is shown in Figure 4 .
A simple chemometric evaluation is applied with Principal Component Analysis (PCA) performed using the 2.60 version of Pirouette software (Infometrix, Woodinville, WA, USA) (see the BEEBOOK manuscript on statistical guidelines for more information on using PCA, Pirk et al., 2013 ). Only the two most characteristic negative ion markers of each sample are selected and expressed as the intensities of these individual ions (variables). The data are preprocessed using auto scale and analyzed using PCA.
Due to the prevalence of acid compounds, the negative ion mode fingerprints result in the clearest discrimination between the groups of propolis samples. This pattern was confirmed by subsequent studies of propolis fingerprinting conducted by Sawaya, da Silva, Cunha, and Marcucci ( 2011 ).
Infuse these solutions directly into the ESI-source of a hybrid high resolution and high-accuracy (5 ppm) Micromass Q-TOF mass spectrometer, via a syringe pump (Harvard Apparatus) at a flow rate of 15 μl/min. The MS conditions should be capillary −3.0 kV, cone 30 V.
MS fingerprinting is a qualitative analytical tool used to discern between different types of propolis and to compare the composition of propolis samples to those of plant resins. MS fingerprints are proposed as characteristic of the composition of samples and can be used as a guide for their therapeutic uses. The method used in one study (Sawaya et al., 2004 ) was only slightly modified in the subsequent applications and can be considered as the standard method for propolis extraction for MS fingerprinting.
3.6. NMR analysis of propolis
3.6.1. Introduction Since its discovery, the phenomenon of Nuclear Magnetic Resonance (NMR) has been widely exploited as a research tool in analytical laboratories throughout the world. NMR spectroscopy is used to study the structure of molecules (Kwan & Huang, 2008). It also is well known that NMR can be used to analyze complex mixtures such as herbal extracts, foods, biological fluids, etc. (Forseth & Schroeder, 2011). In particular, NMR is used increasingly in the evaluation of food and in the quality assurance of natural products, although all its potential has not been fully exploited. The amount of information available in an NMR spectrum and the ease of sample preparation make this spectroscopic technique very attractive for the assessment of product quality. One of the main advantages of this technique over that of other methods is its ability to furnish structural and quantitative information on a wide range of chemical species in a single NMR experiment. The mixture analysis by NMR is complex, but potentially very informative (Lin & Shapiro, 1997). In recent years, the use of much higher magnetic fields and the greater sensitivity and spectral resolution that they bring, have stimulated interest in 1D and 2D NMR spectroscopy as a routine method for the analysis of complex mixtures (Charlton, Farrington, & Brereton, 2002; Fan, 1996). There are two main strategies for analyzing mixtures via NMR: (a) separate components of the mixture prior to NMR analysis; and (b) analyze the mixture as it is. The first strategy is used when the goal of the work is the characterization of an isolated compound and it is not the subject of this discussion. The second strategy allows one to obtain an overall image of the mixture in question, without any further type of pre-treatment of the sample, except the eventual solubilization in a suitable deuterated solvent. The obtained spectra will be considered as chemical fingerprints of the product under investigation. In this case, the analysis of the spectra, that usually appear very complex, requires tools for the pre-treatment of the signal and for the analysis of the results, normally based on multivariate statistical techniques (Papotti, Bertelli, Plessi, & Rossi, 2010).
3.6.2. Sample preparation Since propolis is a solid material, it requires an initial extraction procedure using 70% ethanol (see Section 3.1.1). Obviously, if the extract is analyzed as is, very intense signals related to the solvent will be present in the obtained spectra. To avoid this problem, it is preferable to eliminate the solvents under a light nitrogen stream operating at low temperature. This procedure can be conducted directly in NMR tubes, and by dissolving the solid residue in an appropriate volume of the selected deuterated solvents. The most important thing to remember when choosing the most suitable solvent is that if D 2 O is chosen, all the signals relating to alcoholic, phenolic or carboxylic hydroxyls, that are very abundant in propolis, will be lost in the spectrum. If one is interested to observe the signals related to these functional groups, a solvent that does not exchange deuterium with hydroxyls should be used. The most suitable in the case of propolis is the DMSO-d 6 (deuterated dimetyl sulfoxyde) (Papotti et al., 2010). There is no ideal ratio of propolis extract and the amount of solvent used; each one must find what works best in each case. (1) Transfer 1ml of propolis extract (see 3.1) to an NMR tube and evaporate to dryness at room temperature using a flow of nitrogen gas. (2) Dissolve the dry residue in 0.5 ml of methyl sulphoxide-d6 (DMSOd6). (3) Add 20 μl of tetramethylsilane (TMS) as a reference compound. (4) Use the sample immediately for NMR experiments.